10 BAGGER GEM HERE EASILY AND POTENTIAL BUYOUT CANDIDATE
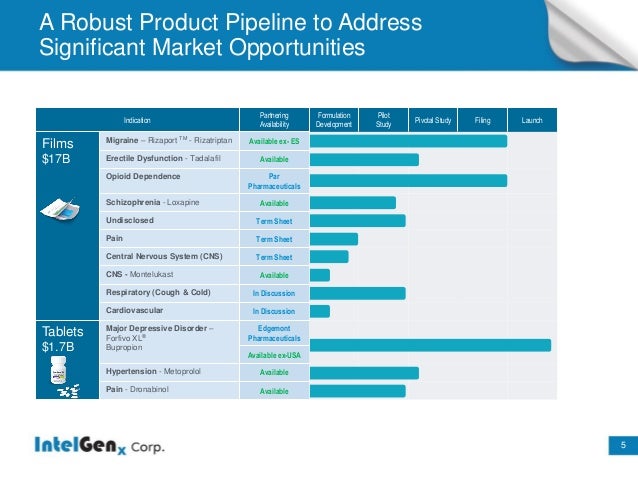
Undiscovered Intelgenx (IGXT) is definitely one of the most attractive and undervalued stock in the Biotech Sector . The Company has already 2 Drugs approved and another 2 Big Drugs close to FDA approval .BIG Partnership News could come any day for up to 3 Drugs for which IGXT got a Therm Sheet from a Global Pharma Company and there is another partnering discussion ongoing for 2 Drugs (see pipeline chart below) .
IGXT has a ridiculous Market Cap of less than $32 Million which is a Big Joke for a Company with such a MEGA Product Pipeline , this Co has everything to be the next 10 Bagger minimum .GL
Intelgenx (IGXT)
Market Cap: $31.8 Million
Cash: $2.1 Million
Price: $0.49
Burn-Rate: $400k per Quarter
Shares Out : 63.6 Million
UPCOMING MILESTONES :
Big Partnership imminent :
•Announced a development and commercialization term sheet with a global pharmaceutical company for up to three products. If entered into, IntelGenx expects the definitive agreement to be finalized in the second quarter of 2016
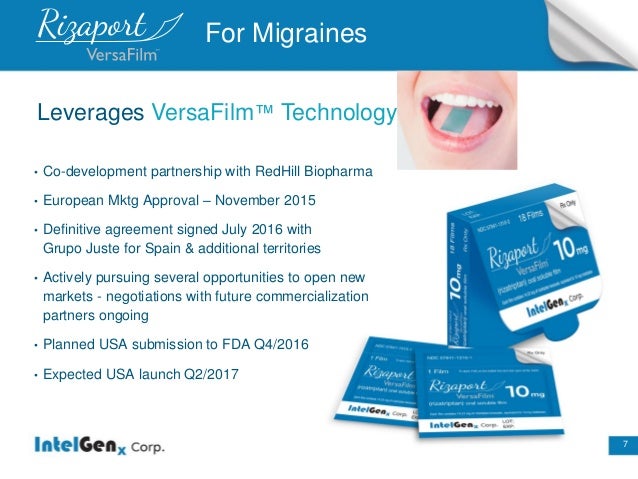
Product :Rizaport (Migraine)
•European Mktg Approval–November 2015
•Planned USA submission to FDA Q4/2016
•Expected USA launch Q2/2017
Product :Tadalafil (Erectile Dysfunction) a better Oral thin-film version of Blockbuster Drug Cialis
•505(b)(2) USA NDA submission in Q4/2016
•Expected USA launch Q4/2017
Product :Indicated for Opioid Dependence
•Awaiting FDA approval
•According to IMS data, the oral film market for opioid dependence was worth more than $1.4B US in 2014
IntelGenx Corp., today announced the recent initiation of a phase 1 clinical trial of montelukast, a unique drug repurposing opportunity for the treatment of degenerative diseases of the brain, such as: mild cognitive impairment and Alzheimers disease, the most prominent form of dementia. IntelGenx expects results from the phase 1 trial to be available in September 2016.

|







