| (ETON)..Market Cap $133 M / 2 FDA decisions imminent for attractive products first one on July 11 and next one on October 21 / HUGE late stage pipeline with multiple milestones in near-term milestones (read below) /undiscovered Ultra low float stock with multi bagger potential here : GLTA
Eton Pharma (ETON)
Market-cap: $130,7 Million
Cash: $19,5 Million
Price: $7,42
Shares Out: 17,6 Million
Presentation May 2019
ir.etonpharma.com
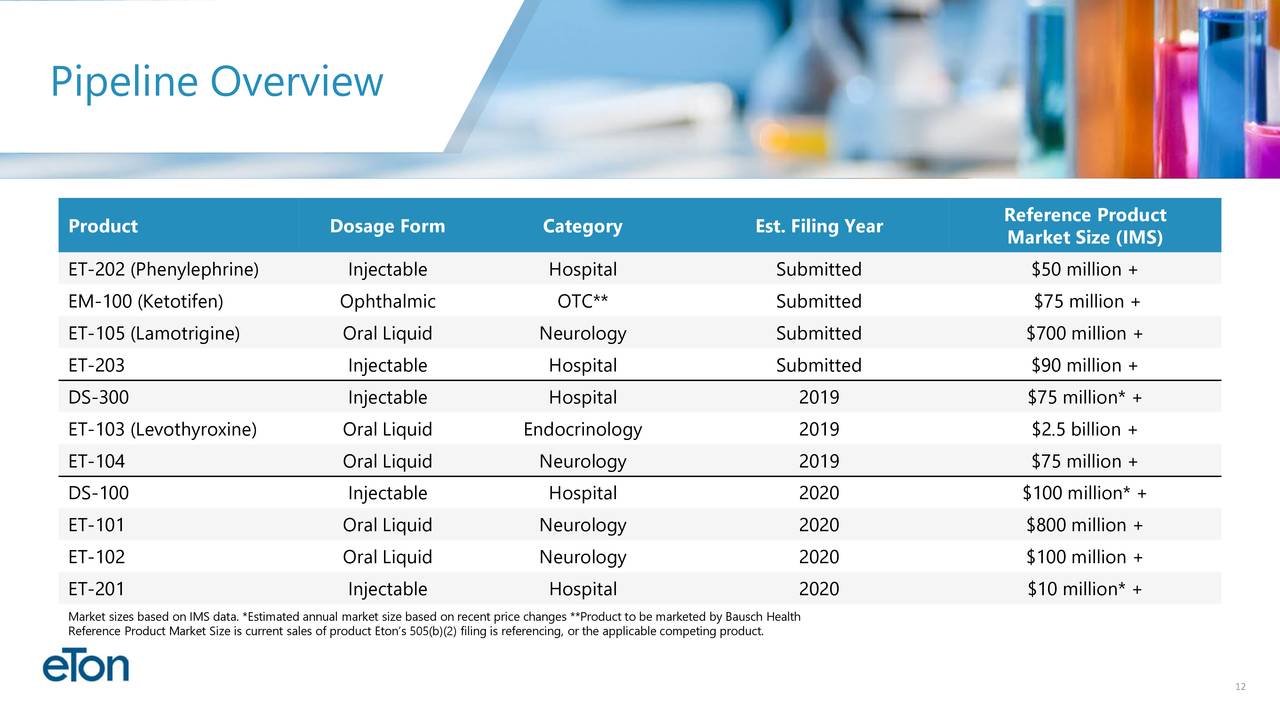
•Diversified pipeline of 11 products under development. Four products submitted to the FDA,three additional NDA’s expected to be submitted in 2019
•Expect to become commercial revenue company in 2019
Products:
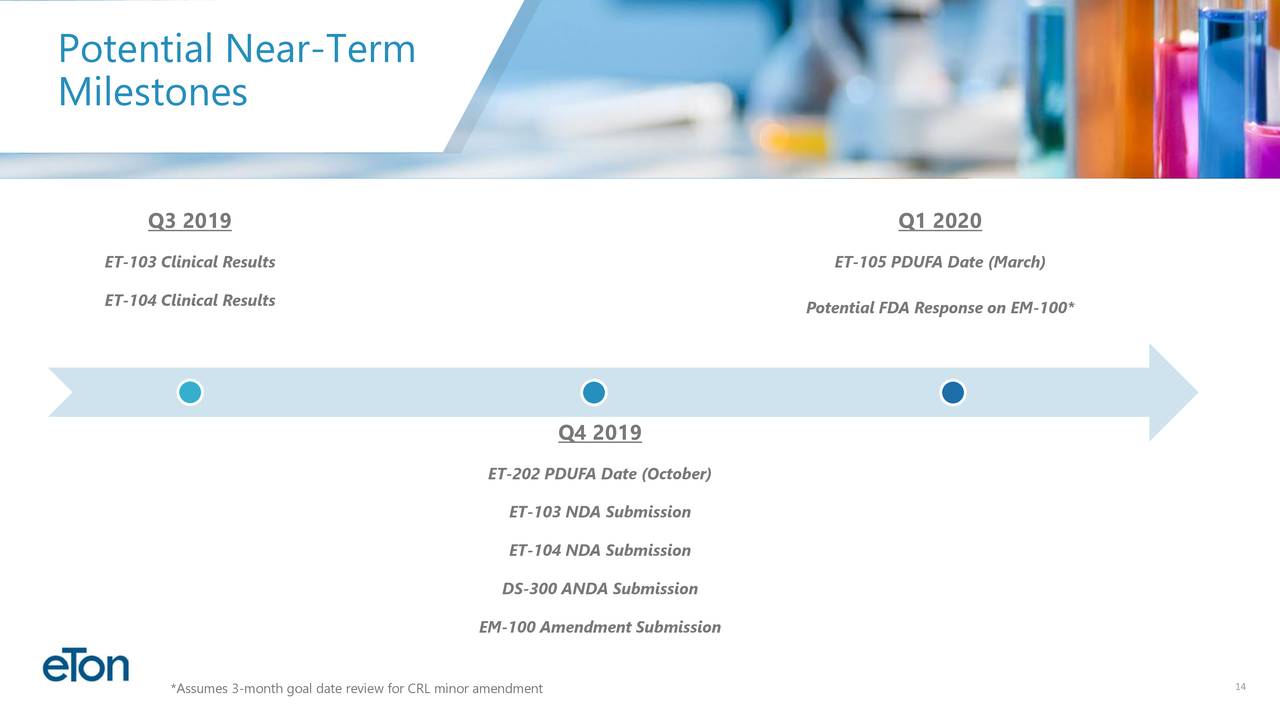
EM-100. EM-100, Eton’s preservative-free ophthalmic solution for allergic conjunctivitis has been assigned a target action date of July 11, 2019. Bausch Health will be responsible for all remaining regulatory and commercial activities surrounding the product. Eton is entitled to a milestone payment upon product launch and a royalty on commercial sales.
ET-202. Eton has initiated launch preparations for ET-202, Eton’s ready-to-use injectable formulation of phenylephrine. If approved on its PDUFA date of October 21, 2019, Eton anticipates launching the product in the fourth quarter of 2019. Eton believes the addressable phenylephrine market for ET-202 is more than 10 million units annually.
ET-105 is an innovative patent-pending formulation of lamotrigine that will be delivered to patients as an oral liquid. Aucta submitted the product’s New Drug Application (NDA) to the FDA in May 2019 and is seeking approval as an epilepsy treatment to be used as an adjunct therapy for partial seizures, primary generalized tonic-clonic seizures, and generalized seizures of Lennox-Gastaut syndrome in patients two years of age and older.
ET-203. The NDA for ET-203, a ready-to-use formulation of a high-volume injectable product, is expected to be submitted by Eton’s partner by the end of the third quarter of 2019.
ET-104. The bioequivalence study for ET-104, a patent-pending oral suspension pursuing a neurological indication, is ongoing. Eton expects study results in September and, if successful, plans to submit the NDA in the fourth quarter of 2019.
DS-300. Due to a third-party approval of another NDA product containing DS-300’s active ingredient, the FDA has notified Eton’s development partner that DS-300 no longer qualifies for the NDA regulatory pathway and will be required to follow the ANDA regulatory pathway. Eton is pursuing the FDA’s process to appeal the decision. If the appeal is unsuccessful, Eton plans to re-submit DS-300 as an ANDA later this year.
ET-103. The bioequivalence study for ET-103, a liquid formulation of levothyroxine, is ongoing. Eton expects study results in September and, if successful, plans to submit the NDA in the fourth quarter of 2019.
DS-100. Eton has an FDA meeting scheduled for August 2019 to discuss DS-100’s clinical pathway. If successful, Eton anticipates submitting the product’s NDA in 2020.
ET-101. Development activities are ongoing for ET-101, an innovative oral liquid neurology product. Eton currently expects to submit the product’s NDA in 2020
ET-102. Development activities are ongoing for ET-102, an innovative oral liquid neurology product. Eton currently expects to submit the product’s NDA in 2020
ET-201. Development activities are ongoing for ET-201, an injectable product currently approved in Europe. Eton expects to submit the product’s NDA in 2020.
Largest Shareholders
Harrow Health, Inc...3,500,000
Peter A. Appel...1,249,329 ´
Sean Brynjelsen, MBA...1,034,940
Mark L. Baum...794,745
Charles J. Casamento, MBA...60,420
Paul V. Maier, MBA...59,745
Norbert G. Riedel, PhD ...59,745
Wilson W. Troutman, CPA...5,000
thefly.com
Eton Pharmaceuticals price target raised to $18 from $15 at H.C. Wainwright H.C. Wainwright analyst Raghuram Selvaraju raised his price target for Eton Pharmaceuticals to $18 from $15 after the company in-licensed ET-105, a patent-pending formulation of lamotrigine designed to be delivered to patients as an oral liquid, from the privately-held Aucta Pharmaceuticals. The analyst believes the in-licensing of ET-105 meaningfully expands Eton's "stable" of neurology-focused product candidates. He reiterates a Buy rating on the shares.
 |
|







