The bull case for TRIL from "Seeking Alpha"
The CD47 Space Is Heating Up And Trillium Stands To Benefit
Nov. 5, 2017 10:19 PM ET
|
About: Trillium Therapeutics Inc. (TRIL)

Jonathan Faison
Long only, biotech, event-driven
(5,806 followers)
Summary
Shares have jumped 50% since my recent write-up.
ASH data appears quite promising and validates the company's unique approach.
I believe highs last seen in 2015 could be surpassed if momentum continues through year-end.
I wouldn't be surprised to see a partner come on board in the near to medium term.
Readers interested in the story who have done their due diligence should make an initial pilot purchase or add to their positions in the near term.
Shares of Trillium Therapeutics ( TRIL) have risen by 50% since I suggested a run-up into year-end was in the cards.
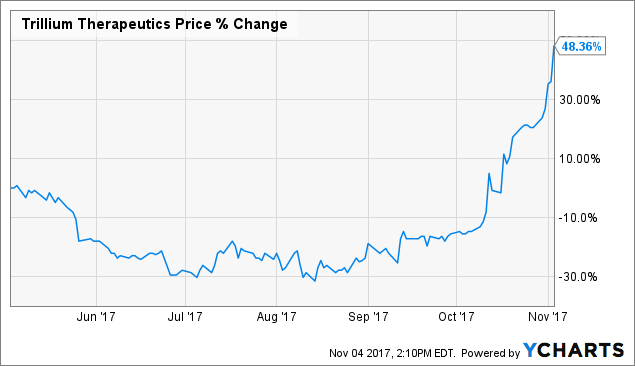 TRIL data by YCharts TRIL data by YCharts
Keys to the bullish thesis include the following:
I pointed out that CD47 was and continues to be an attractive oncology target, with preclinical efficacy established across a variety of indications and synergy with other immunotherapies likely. Prior deals and collaborations in the space also hinted at the value here, with Trillium poised to capitalize with TTI-621 (SIRPaFc fusion protein targeting CD47).Wall Street appeared to write off the stock in 2016 when two of five patients in the highest dose cohort of a phase 1 study experienced dose-limiting toxicity (Grade 3 elevated liver enzymes and Grade 4 thrombocytopenia). However, additional data clarified that this transient thrombocytopenia often lessens after multiple infusions, suggesting that there could be an opportunity to increase exposure to the study drug in patients after the initial dose.I referenced the $30 million June financing as a positive development, with significant positions owned by Growth Equity Opportunities V (9.9%) and Cormorant AM (5.66%). I also pointed out that Baker Brothers Advisors, Tekla Capital Management and NEA Management all own stakes as well.Readers were advised that preliminary data from the TTI-621 phase 2a intratumoral dose escalation study in solid tumors and additional clinical data from the phase 1b intravenous study could drive near-term upside.
The title of their ASH abstract is "TTI-621 (SIRPaFc), an Immune Checkpoint Inhibitor Blocking the CD47 "Do Not Eat" Signal, Induces Objective Responses in Patients with Advanced, Relapsed/Refractory Diffuse Large B-Cell Lymphoma (DLBCL)." The presentation will take place December 11th from 6:00 PM to 8:00 PM.
In the phase 1 open-label study, weekly IV infusions of TTI-621 were evaluated in several cohorts of adult patients with advanced disease (relapsed or refractory malignancies). These patients had already progressed on rituximab or other standard therapy - they were administered 0.2 mg/kg/week TTI-621 monotherapy or 0.1 mg/kg TTI-621 in combination with 375 mg/m2/week rituximab for a period of 8 weeks. From there, TTI-621 monotherapy was allowed.
At June 1st, 14 DLBCL patients were evaluable, 6 having received monotherapy and 8 who were administered TTI-621 plus rituximab. Objective responses in 5/14 (36%) patients were encouraging, which included 1 of 6 monotherapy-treated patients and 4 of 8 treated with the combination (a higher 50%). Notably, two patients had complete responses (CR) which were comprised of a monotherapy-treated patient whose CR continued after 20 weeks and a combination treated patient whose CR continued after 12 weeks. Three patients exhibited partial responses. Importantly, treatment was well-tolerated (monotherapy and combination).
A second abstract reveals how researchers were able to demonstrate that CD47 blockade with TTI-621 increases phagocytosis of lymphoma cells, including those with lower phagocytic capabilities such as M(-), M(IL-4) and M(HAGG+IL-1ß) macrophages. The study gives further evidence that combination approaches are warranted across several oncology indications.
While it has certainly been a rollercoaster of a ride for long-time shareholders, armed with recent data, I would not be surprised to see the company's stock price and valuation march steadily past highs last seen in 2015.
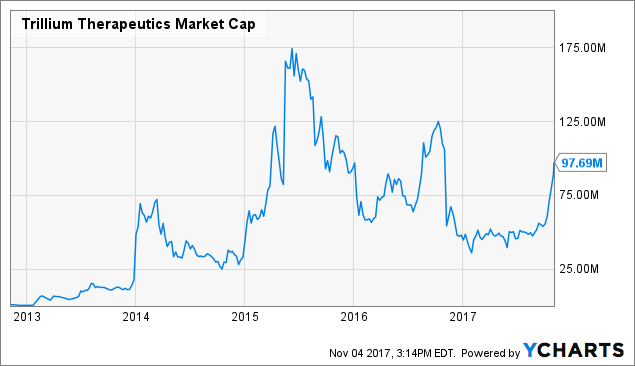 TRIL Market Cap data by YCharts TRIL Market Cap data by YCharts
Another sign that the CD47 space is alive and well is the recent $75 million Series B Financing for Forty Seven Inc., led by Wellington Management Company with participation from existing investors (Clarus, Lightspeed Venture Partners, Sutter Hill Ventures and Google Ventures). Proceeds will be utilized to continue ushering their CD47 antibody Hu5F9-G4 through clinical studies (currently the subject of 5 monotherapy phase 1b and combination therapy phase 1b/2 studies). Current trials will be expanded and new studies begun combining the drug candidate with checkpoint inhibitors, further confirming my belief that this space is heating up. Those involved have obviously liked what they have seen to date.
I remind readers that the company last reported cash and equivalents of $72.6 million, while net loss for the first six months of 2017 totaled $23.1 million. With their cash position accounting for much of their sub $100 million market capitalization, I believe that a revaluation is in order based on the new data and renewed promise for their unique approach. Additionally, I wouldn't be surprised to see a partner come on board in the near to medium term, such as Roche ( OTCQX:RHHBY) or Merck ( MRK). At the same time, there is no need for the company to rush business development, and as data matures along with further updates their negotiating position continues to improve.
Trillium Therapeutics is a Conviction Buy.As a member of the ROTY Contender List, I am aware several followers hold the stock and others have been following the story closely.
Readers interested in the story who have done their due diligence should go and make an initial pilot purchase or add to their positions in the near term. I suggest adopting a "buy the dips" strategy, as I consider the stock cheap even after the recent run-up. The stock appears attractive on multiple time frames, so it is up to readers to determine their plan for profiting and sticking to it. As usual, after significant gains are realized I suggest taking partial profits while retaining shares for continued upside. However, at this point, I would also suggest retaining a larger than usual stake (50% to 75% of original position) based on promising developments and future promise.
After the June financing, the risk of dilution appears to be off the table in the near term. I am further convinced after recent data that a partnership could be forthcoming, which would reduce the risk of dilution even more. However, in the absence of a partnership or other form of funding, capital markets will likely be accessed by the second half of 2018. Safety concerns, although less of an issue than before, are still significant and should be taken into consideration. Further, severe adverse safety events (including the possibility of a clinical hold) should be carefully considered prior to taking a position. Setbacks in ongoing and planned studies (both as a monotherapy and in combination) would negatively impact the stock price as well. Readers would also do well to keep tabs on progress by competition in the space.
Bladerunner
|




